The TMF Reference Model is intended to cover the full scope of the Trial Master File as described in ICH E6 Good Clinical Practice Guidelines. ICH E6 GCP Guidelines include all the content that the sponsor is required to maintain in the sponsor TMF and all the content that the investigator is required to maintain in the investigator TMF; the latter is sometimes referred to as the ‘investigator site file’, the ‘ISF’, or the ‘regulatory binder’.
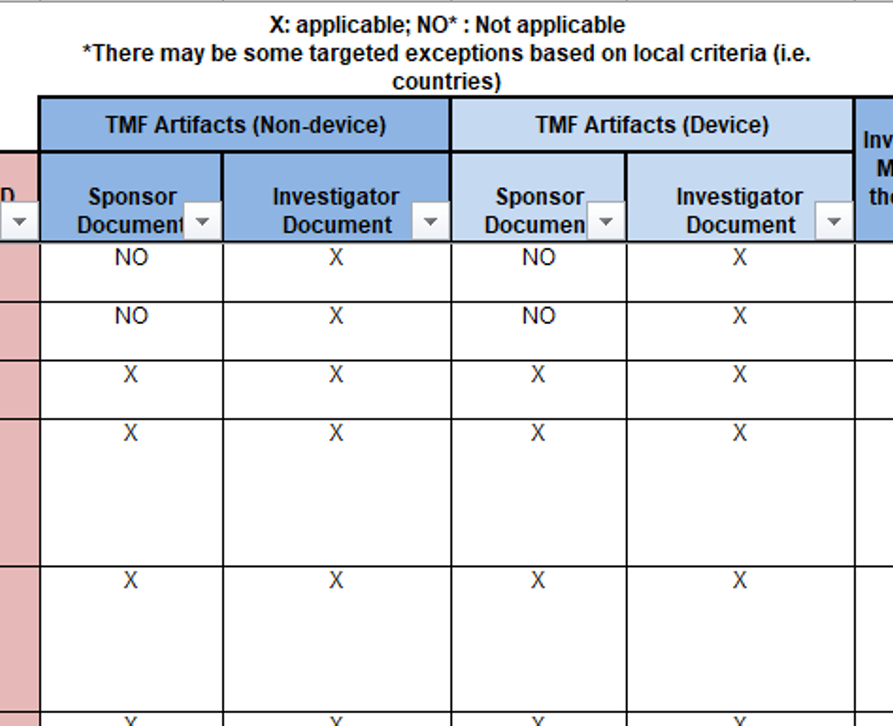 The structure of the TMF Reference Model spreadsheet includes two columns where artifacts are designated as being sponsor artifacts or investigator artifacts. These two columns are shown for non-device trials (columns N and O) and for device trials (columns P and Q). Any suggestion that the Reference Model does not include investigator site documents is incorrect.
The structure of the TMF Reference Model spreadsheet includes two columns where artifacts are designated as being sponsor artifacts or investigator artifacts. These two columns are shown for non-device trials (columns N and O) and for device trials (columns P and Q). Any suggestion that the Reference Model does not include investigator site documents is incorrect.
The TMF Reference Model project team have received several comments over the years that suggest the Model can be difficult to apply to the investigator TMF and that it is not consistent with file structures that are typically used. The Steering Committee recently (January 2018) agreed to be involved with an industry initiative being organized by MAGI to introduce improvements. Representatives from the TMF Reference Model project team will participate in the MAGI project to produce an investigator TMF Reference Model that will be available as a standalone product from MAGI. Once this work is complete, it will be fully integrated into an update of the TMF Reference Model; we want to continue making available a single consistent Model across the sponsor and investigator TMFs. The objectives of this initiative are:
- to identify any missing artifacts relevant to the investigator TMF;
- to identify sub-artifacts that are relevant to the investigator TMF e.g. examples of source data that might be found in the investigator TMF;
- to review the structure of the content to ensure alignment with regulatory expectations and industry best practice;
- to ensure the content reflects requirements and best practice globally.
Sholeh Ehdaivand (sholeh.ehdaivand@lmkclinicalresearch.com) is leading the MAGI project. The team is currently developing a site reference model that will be mapped to the (DIA) TMF Reference Model.
Is there any update? Is there a ISF index that we can use with our sites for the Reg/ISF Binders?